Ethical Obligation Or A Drug With Dangerous Adverse Effects That Need To Be Hidden?
Executive Summary
- Ethical considerations are used as an excuse for pharmaceutical companies to end clinical trials early.
- This is done to hide the adverse effects of the drug.

Introduction
We have observed a pattern when hearing that a study must end early because the.
Results were so good, it would be unethical to deny the control group the treatment.
The results are not good, but the pharmaceutical company needs an excuse to end the study early. This article will explain how pharmaceutical companies repeatedly use this tactic.
Our References for This Article
To see our references for this article and related Brightwork articles, visit this link.
Ethical Obligation, Or A Drug With Dangerous Side Effects That Need To Be Hidden?
An excellent example of this is found in the clinical trials of the covid vaccines. The excuse was given that after The FDA granted emergency Use Authorization, the study needed to be halted after only two months. After the FDA provided the EUA, denying the control group the treatment would have been unethical.
The Canadian Covid Care Alliance
The following graphic from the Canadian Covid Care Alliance shows what happened to the study when the control or placebo group was added to the test group after two months.
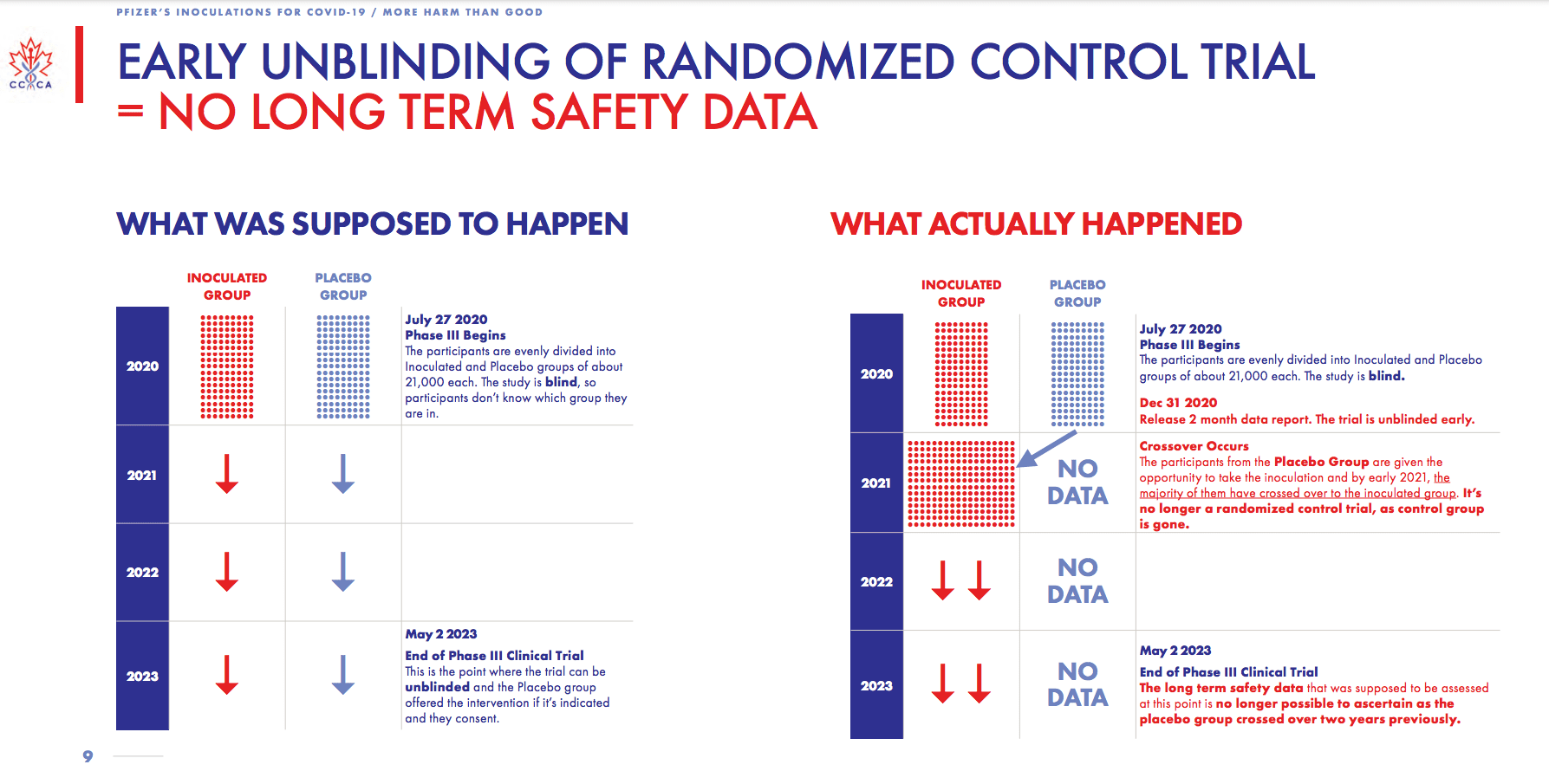
This graphic shows that the placebo group was removed, which begs why the study was unblinded early. Observe the quote from the bottom right corner of the graphic.
This term “ethical obligation” has been used many times in the past and was used again by Dr. Fauci to stop the long-term testing of Remdesivir, a deadly drug. The article ” How Gilead Brought Off the NIH’s Support of Remdesivir ” covers this.
Pharmaceutical Companies Can’t-Wait To End Studies Early
Pharmaceutical companies love abbreviated studies, allowing them to hide the long-term adverse reactions from their drugs more effectively. Drug companies have a special department inside them where they come up with excuses as to why the study needs to be shortened.
Billions of people would take these vaccines. Still, according to the drug companies, they had an “ethical obligation” to stop their study from being able to test the adverse reactions of 18,000 or so people in the control group. Anytime “ethical obligation” is used as logic for ending a clinical trial early, one can be assured that the drug has dangerous side effects.
The ethical obligation was a scam term used to end the study early to bury the vaccine safety problems. It had zero scientific basis and was made up of pharmaceutical companies and supported by the FDA.
Finally, the FDA wanted to approve virtually any vaccine, as they were under tremendous political pressure. This is explained in the article How Emergency Use Authorization For The Covid Vaccines Is A Get Out Of Jail Free Card For the FDA.